STRUCTURE
Page 1 of 1
 STRUCTURE
STRUCTURE
Structure of solids, liquids, gases: of atoms, molecules, chemical bonding: of metals- atomic, microstructure, macrostructure: Grain size, solid solutions, phase diagrams.
 Re: STRUCTURE
Re: STRUCTURE
Categories of Solids Based on the Solid Pack
Solids can be divided into three categories, based on the way the particles that form the solid are packed.
Crystalline Solids are three- dimensional analogs of a brick wall. They have a regular structure, in which the particles pack in a repeating pattern from one edge of the solid to the other.
A crystal or crystalline solid is a solid material whose constituents, such as atoms, molecules or ions, are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions.
The crystal lattice can be thought of as an array of 'small boxes' infinitely repeating in all three spatial directions. Such a unit cell is the smallest unit of volume that contains all of the structural and symmetry information to build-up the macroscopic structure of the lattice by translation.
Crystals have two parts:
1. Lattice - Regular periodic array of points in space.
2. Basis - A group of atoms located at each point of the lattice.
In mineralogy and crystallography, a crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. It describes a highly ordered structure, occurring due to the intrinsic nature of its constituents (parts) to form symmetric patterns.
A crystal's structure and symmetry play a role in determining many of its physical properties, such as:
1. Cleavage - the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye.
2. Electronic band structure - describes those ranges of energy that an electron within the solid may have (called energy bands, allowed bands, or simply bands) and ranges of energy that it may not have (called band gaps or forbidden bands).
3. Optical transparency - describes the behaviour of light in anisotropic media, that is, media (such as crystals) in which light behaves differently depending on which direction the light is propagating. The index of refraction depends on both composition and crystal structure and can be calculated using the Gladstone–Dale relation. Crystals are often naturally anisotropic, and in some media (such as liquid crystals) it is possible to induce anisotropy by applying an external electric field.
The crystal structure of a material (the arrangement of atoms within a given type of crystal) can be described in terms of its unit cell. The unit cell is a small box containing one or more atoms arranged in 3-dimensions. The unit cells stacked in three-dimensional space describe the bulk arrangement of atoms of the crystal. The unit cell is represented in terms of its lattice parameters, which are the lengths of the cell edges (a,b and c) and the angles between them (alpha, beta and gamma), while the positions of the atoms inside the unit cell are described by the set of atomic positions (xi , yi , zi) measured from a lattice point. Commonly, atomic positions are represented in terms of fractional coordinates, relative to the unit cell lengths.
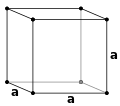
Simple cubic (P)

Body-centered cubic (I)

Face-centered cubic (F)
The atom positions within the unit cell can be calculated through application of symmetry operations to the asymmetric unit. The asymmetric unit refers to the smallest possible occupation of space within the unit cell. This does not, however imply that the entirety of the asymmetric unit must lie within the boundaries of the unit cell. Symmetric transformations of atom positions are calculated from the space group of the crystal structure, and this is usually a black box operation (a device, system or object which can be viewed in terms of its inputs and outputs (or transfer characteristics), without any knowledge of its internal workings. Its implementation is "opaque" (black). Almost anything might be referred to as a black box: a transistor, algorithm, or the human brain.) performed by computer programs. However, manual calculation of the atomic positions within the unit cell can be performed from the asymmetric unit, through the application of the symmetry operators described within the 'International Tables for Crystallography: Volume A'
Amorphous Solids (literally, "solids without form") have a random structure, with little if any long-range order.
Polycrystalline Solids are an aggregate of a large number of small crystals or grains in which the structure is regular, but the crystals or grains are arranged in a random fashion.
Solids can be divided into three categories, based on the way the particles that form the solid are packed.
Crystalline Solids are three- dimensional analogs of a brick wall. They have a regular structure, in which the particles pack in a repeating pattern from one edge of the solid to the other.
A crystal or crystalline solid is a solid material whose constituents, such as atoms, molecules or ions, are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions.
The crystal lattice can be thought of as an array of 'small boxes' infinitely repeating in all three spatial directions. Such a unit cell is the smallest unit of volume that contains all of the structural and symmetry information to build-up the macroscopic structure of the lattice by translation.
Crystals have two parts:
1. Lattice - Regular periodic array of points in space.
2. Basis - A group of atoms located at each point of the lattice.
In mineralogy and crystallography, a crystal structure is a unique arrangement of atoms, ions or molecules in a crystalline liquid or solid. It describes a highly ordered structure, occurring due to the intrinsic nature of its constituents (parts) to form symmetric patterns.
A crystal's structure and symmetry play a role in determining many of its physical properties, such as:
1. Cleavage - the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye.
2. Electronic band structure - describes those ranges of energy that an electron within the solid may have (called energy bands, allowed bands, or simply bands) and ranges of energy that it may not have (called band gaps or forbidden bands).
3. Optical transparency - describes the behaviour of light in anisotropic media, that is, media (such as crystals) in which light behaves differently depending on which direction the light is propagating. The index of refraction depends on both composition and crystal structure and can be calculated using the Gladstone–Dale relation. Crystals are often naturally anisotropic, and in some media (such as liquid crystals) it is possible to induce anisotropy by applying an external electric field.
The crystal structure of a material (the arrangement of atoms within a given type of crystal) can be described in terms of its unit cell. The unit cell is a small box containing one or more atoms arranged in 3-dimensions. The unit cells stacked in three-dimensional space describe the bulk arrangement of atoms of the crystal. The unit cell is represented in terms of its lattice parameters, which are the lengths of the cell edges (a,b and c) and the angles between them (alpha, beta and gamma), while the positions of the atoms inside the unit cell are described by the set of atomic positions (xi , yi , zi) measured from a lattice point. Commonly, atomic positions are represented in terms of fractional coordinates, relative to the unit cell lengths.

Simple cubic (P)

Body-centered cubic (I)

Face-centered cubic (F)
The atom positions within the unit cell can be calculated through application of symmetry operations to the asymmetric unit. The asymmetric unit refers to the smallest possible occupation of space within the unit cell. This does not, however imply that the entirety of the asymmetric unit must lie within the boundaries of the unit cell. Symmetric transformations of atom positions are calculated from the space group of the crystal structure, and this is usually a black box operation (a device, system or object which can be viewed in terms of its inputs and outputs (or transfer characteristics), without any knowledge of its internal workings. Its implementation is "opaque" (black). Almost anything might be referred to as a black box: a transistor, algorithm, or the human brain.) performed by computer programs. However, manual calculation of the atomic positions within the unit cell can be performed from the asymmetric unit, through the application of the symmetry operators described within the 'International Tables for Crystallography: Volume A'
Amorphous Solids (literally, "solids without form") have a random structure, with little if any long-range order.
Polycrystalline Solids are an aggregate of a large number of small crystals or grains in which the structure is regular, but the crystals or grains are arranged in a random fashion.
Page 1 of 1
Permissions in this forum:
You cannot reply to topics in this forum|
|
|
